NICE (Neutron-Induced Carcinogenic Effects)
| History |
NNS |
Carcinogenesis |
Published Research Papers by the
NICE team F. Mathew, C. Chilian, L. Montgomery, and J. Kildea. Development of a passive gold-foil nested neutron spectrometer to validate the active current-mode He-3 measurements in a high neutron fluence rate radiotherapy environment. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 985:164662, 2020. L. Montgomery, A. Landry, G. Al Makdessi, F. Mathew, and J. Kildea. A novel MLEM stopping criterion for unfolding neutron fluence spectra in radiation therapy. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment, 957:163400, 2020. C. Lund, G. Famulari, L. Montgomery, and J. Kildea. A microdosimetric analysis of the interactions of mono-energetic neutrons with human tissue. Physica Medica, 73:29–42, 2020. L. Montgomery, M. Evans, L. Liang, R. Maglieri, and J. Kildea. The effect of the flattening filter on photoneutron production at 10 MV in the Varian truebeam linear accelerator. Medical physics (doi.org/10.1002/mp.13148), 2018. F. Ali, J. Atanackovic, C. Boyer, A. Festarini, J. Kildea, L. Paterson, R. Rogge, M. Stuart, and R. B. Richardson. Dosimetric and microdosimetric analyses for blood exposed to reactor- derived thermal neutrons. Journal of Radiological Protection, 2018. J. Kildea. The Canadian neutron-induced carcinogenic effects research program-a research program to investigate neutron relative biological effectiveness for carcinogenesis with a particular focus on secondary (by-product) neutrons in high-energy radiation therapy. Radiation Environment Medicine, 6(2):55–61, 2017. R. Maglieri, A. Licea, M. Evans, J. Seuntjens, and J. Kildea. Measuring neutron spectra in radiotherapy using the Nested Neutron Spectrometer. Medical physics, 42(11):6162–6169, 2015.
Students Rafael Khatchadourian, Robert
Maglieri and Georges Al Makdessi were
supported by grants from the Canadian
Nuclear Safety Commission. The research
of Georges Al Makdessi and Robert Maglieri was
partially supported by MPRTN/CREATE.
Laura Paterson's studies are supported by Canadian Nuclear
Laboratories. Industrial Partners: We have an
ongoing collaboration with Canadian
Nuclear Laboratories and Detec Inc.
specifically for this project. Project Description: The NICE research project aims to
improve our understanding of the biophysical
effects surrounding radiation dose deposition
in human tissue using neutrons. Ionizing radiation is a potent
carcinogen that is encountered in our natural
and artificial environments. We cannot avoid
it. Thankfully, radiological protection laws
and regulations protect individuals from the
carcinogenic risk that excess ionizing
radiation poses. However, while these measures
guard against unjustified use of radiation,
they cannot protect three distinct
populations:
Accordingly, any improvement to our
understanding of radiation-induced
carcinogenesis will help clinicians,
policy-makers, and first responders make
informed decisions to protect these
populations. In our research program, we are working
to improve our understanding of the etiology
of radiation-induced cancer by studying
neutrons. We are focusing on neutrons because
their effectiveness at causing cancer is known
to be energy-dependent, which suggests an
underlying energy-dependent mechanism by which
neutrons damage DNA. We are examining the
biophysics involved using a combination of
neutron spectral measurements (physics), Monte
Carlo modelling (physics and chemistry), and
cell irradiation experiments (biology).
Specifically, we are aiming to predict the
relative mutation signatures that neutrons of
different energies will induce in the DNA of
irradiated cells. Using the neutron sources
available to us (radiotherapy accelerators,
reactors, and neutron generators), we will
experimentally test our predictions by
irradiating cells in vitro and examining the
mutational signatures induced in them using a
novel single-cell DNA sequencing protocol that
our group is pioneering. Our combination of
modelling and experiment will provide us with
unique insight into the biophysical action of
ionizing radiation. The results of our research should be of interest to researchers in the radiation medicine, space travel, and nuclear emergency preparedness fields, and our program will provide a rich multi-disciplinary environment to train the next generation of Canadian radiation biology and radiation protection researchers.
 Proton therapy bunker at the Skandion Clinic, Uppsala, Sweden. Our team measured the neutron spectrum around one of the Skandion proton beams in April 2015.
Following from Rafael's studies, Robert
Maglieri joined our research group for his
M.Sc. research and helped innovate the use of
a new Canadian-made neutron spectrum, the Nested
Neutron Spectrometer (NNS) (Detec Inc.,
Gatineau, Quebec) for use in radiotherapy.
Robert adopted the Maximum-likelihood,
expectation-maximization (MLEM) deconvolution
method to unfold raw NNS data.  Schematic cross section of the cylindrical NNS system showing the central He-3 detector and all seven moderators. The NNS 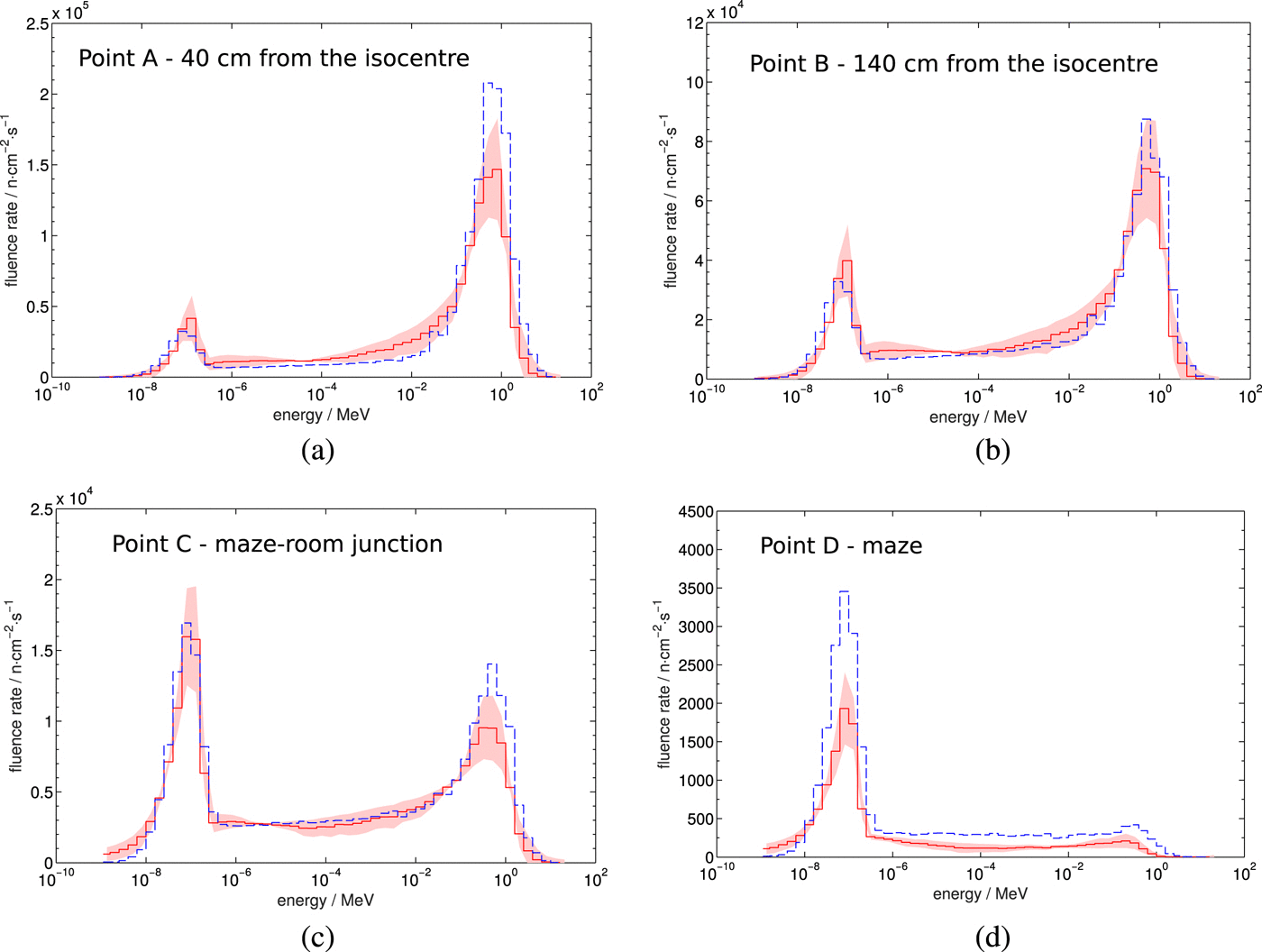
Measured (solid) and simulated (dashed)
neutron spectra in the bunker of a Varian 21EX
linac operated at
18 MV. The shaded region shows the statistical uncertainty associated with the measurements. From
Neutron Spectral Measurements to Studying
Neutron-induced Carcinogenesis Our work with the NNS has provided us with the experience and the motivation to attempt to convert neutron spectral measurements into biologically-meaningful dose estimates. We have shown that we can reliably measure neutron spectra, but, due to the poor understanding of neutron-induced carcinogenesis, we are unable to use these spectra to advise physicians or patients regarding biological damage (carcinogenesis). With this in mind we have formed a
collaboration between McGill, Canadian
Nuclear Laboratories (Chalk River,
Ontario), the Canadian
Nuclear Safety Commission, and Detec Inc.
to investigate neutron carcinogenesis through
a combination of measurements and Monte Carlo
modelling. Our approach is similar to that of
the European ANDANTE
project but it underpinned by our new
measurement technique using the NNS and the
excellent
radio-biological facilities at Chalk
River.  Some members of the McGill-CNL collaboration on a visit to CNL's Chalk River laboratories in October, 2015 The problem summarized:
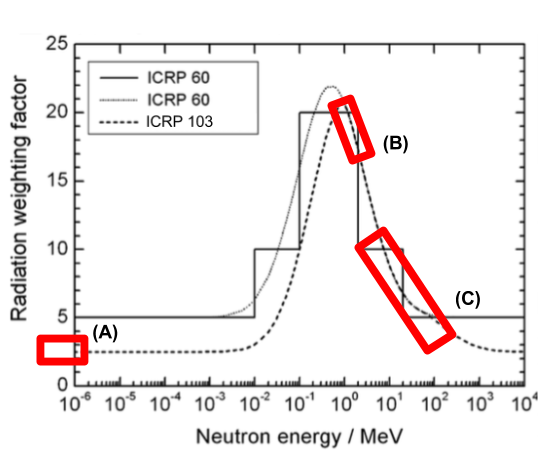
The ICRP radiation
weighting factors for neutrons. Red areas show
approximate Over the next few years we aim to:
The figure below graphically describes
the objectives of the NICE project. Watch this
space!! 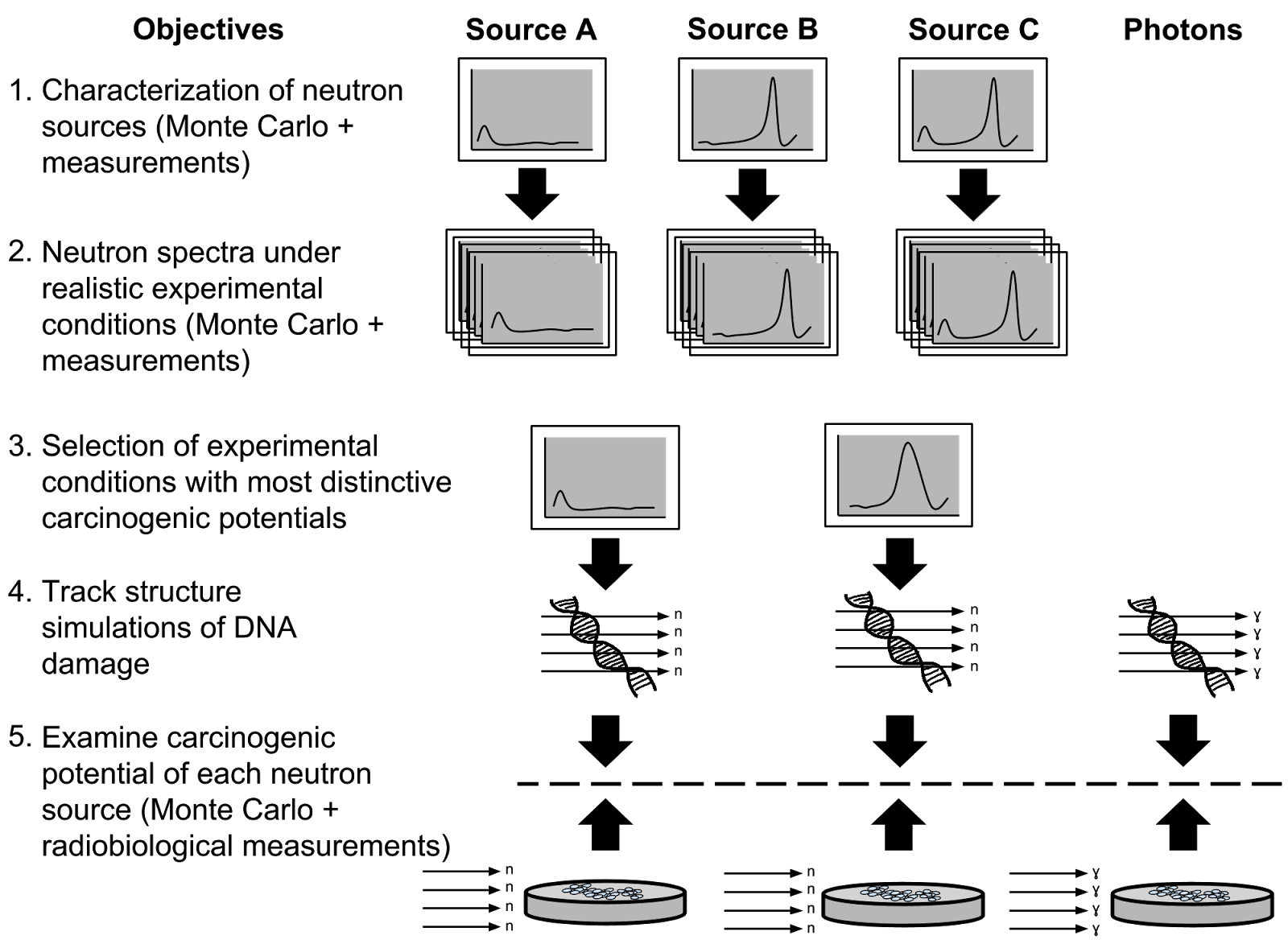 Objectives of the NICE project to investigate the biophysics underlying neutron DNA damage. |